The University of Oxford, Oxford COVID-19 Vaccine Centre's researchers have already begun recruiting human volunteers for the next phase in human trials of a COVID-19 vaccine trial in humans. Image credit: The Oxford COVID-19 Vaccine Centre
The coronavirus that causes COVID-19 disease is still hitting globally and it seems like we can never return to the normal stage until we design and develop an antiviral drug. Researchers from different organizations, health workers, scientific institutions, and universities are working together so they can develop the antidote of COVID-19 as soon as possible. Due to some peculiarity of coronavirus, researchers are holding back.
But now it seems like we may have an antiviral drug of COVID-19 in the near future.
Earlier in April 2020, researchers at Oxford COVID-19 Vaccine Centre started their phase I of the vaccine trial. They tested the vaccine in about 1,110 and immunisations have been completed and follow-up is currently ongoing.
Now, the team is about to start their phase II and III trial of the COVID-19 antiviral vaccine.
Participants in both the Phase II and Phase III groups will receive one or two doses of either the ChAdOx1 nCoV-19 vaccine or a licensed vaccine, MenACWY. Vaccine MenACWY will be used as a 'control' for comparison.
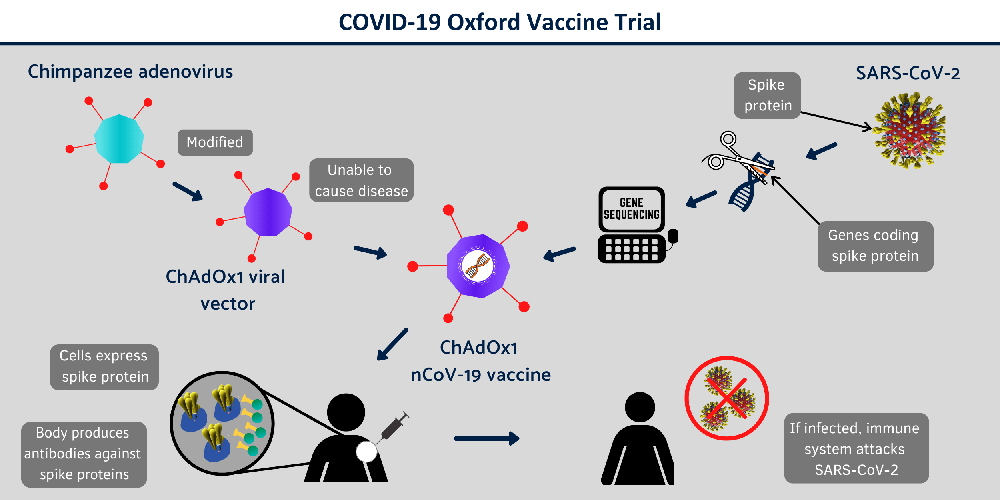
Here, ChAdOx1 nCoV-19 is made from a virus called ChAdOx1, which is a weakened version of a common cold virus, adenovirus. This adenovirus is the same virus that causes infections in chimpanzees. This virus has been genetically changed so that it is impossible for it to replicate in humans.
For their Phase II and III studies, they will enroll up to 10,260 adults and children. Particularly, at phase II they will expand the age range of people the vaccine is assessed in. They will test the vaccine on the people of the varied age groups of age 56-69, age over 70, and age between 5-12 years.
For these age groups, researchers will track the immune response to the vaccine and observe if there is variation in how well the immune system responds in older people or children.
If the result from phase II appears as we required, the trail will enter into phase III.
In phase III of the study, researchers will be assessing how the vaccine works in a large number of people over the age of 18 and how well the vaccine works to prevent people from becoming infected and unwell with COVID-19.
"The clinical studies are progressing very well and we are now initiating studies to evaluate how well the vaccine induces immune responses in older adults, and to test whether it can provide protection in the wider population. We are very grateful to the huge support of the trial volunteers in helping test whether this new vaccine could protect humans against the pandemic coronavirus," said Professor Andrew Pollard, head of the Oxford Vaccine Group.
For this, researchers added the genetic materials from the COVID-19 virus (SARS-CoV-2) called Spike glycoprotein (S), to the ChAdOx1 construct. S-protein is usually found on the surface of SARS-CoV-2 and researchers already found that this protein plays an essential role in the infection pathway of the SARS-CoV-2 virus by binding ACE2 receptors on human cells. Binding of S-protein with ACE2 receptors on human, SARS-CoV-2 coronavirus will enter to the human cells and cause an infection.
Now researchers at the University of Oxford will vaccinate the infected peoples with ChAdOx1 nCoV-19. They are hoping that testing with ChAdOx1 nCoV-19, the human body will recognise and develop an immune response to the Spike protein that will help stop the SARS-CoV-2 virus from entering human cells and therefore prevent infection.
Another vaccine, MenACWY, is being used as an 'active control' vaccine in this study, to help us understand participants' responses to ChAdOx1 nCoV-19. This means MenACWY is simply a controlling and tracking agent for the side effects that may be developed by ChAdOx1 nCoV-19.
To record any symptoms experienced, some participants will be given an E-diary and they will record their experience for 7 days after receiving the vaccine. This record will go on for 3 weeks if they feel unwell. Researchers will also conduct a weekly survey where participants will be asked to complete about any household exposure to COVID-19.
After vaccinated with the antiviral drugs, participants will be in a series of short follow-up visits from the team where the team will check participants' observations, take a blood sample and review the completed E-diary and questionnaire and these blood samples will be used to assess the immune response to the vaccine.
Researchers are hopeful that this will work but if the result goes against us then the team would review progress, examine alternative approaches, such as using different numbers of doses, and would potentially stop the program.
Interested individuals can volunteer to participate in the COVID-19 vaccine website.